
This year marks the centenary of the first time that the French-Canadian microbiologist Félix d’Herelle began using specific viruses to combat bacterial diseases. The use of these bacteriophages, or “phages”, became known as phage therapy, and research on them has continued up to the present day.
A brief look at history reminds us that, in 1919, Félix d’Herelle used phages to treat dysentery in children, and two years later Richard Bruybogne and Joseph Maisin published the first article demonstrating the effectiveness of phage therapy in treating Staphylococcus infections.
In the following decades, studies on phages flourished in Eastern Europe. However, in most of the Western world, phage therapy did not enjoy the same success – possibly due to limited scientific interest, as it coincided with Alexander Fleming’s discovery of penicillin in 1928. That discovery marked the beginning of antibiotic therapy, which remains the most widespread method of fighting bacterial infection to this day.
A century later, as certain bacteria have developed resistance to antibiotics, it seems that phage therapy may have a second chance to successfully treat bacterial diseases.
What are bacteriophages and how do they work in aquaculture?
Bacteriophagues are ubiquitous – they can be found almost everywhere. Fortunately for aquaculture, the marine environment is one of the richest sources of these viruses. It is estimated that there may be around 10⁹ viral particles per millilitre of seawater, with up to 70% of marine bacteria infected by phages.
This abundance suggests that phage therapy could become a highly effective tool in aquaculture in the coming years, as early trials have already shown.
With few exceptions, phage therapy remains one of the “great unknowns” in aquaculture, and there is a clear need for more research groups to engage in this field to expand its use beyond the current limited applications.
To bring this topic closer to misPeces.ocm readers, we have reviewed several international studies exploring the feasibility of phage therapy in commonly farmed aquatic species.
How do phages develop their bactericidal action?
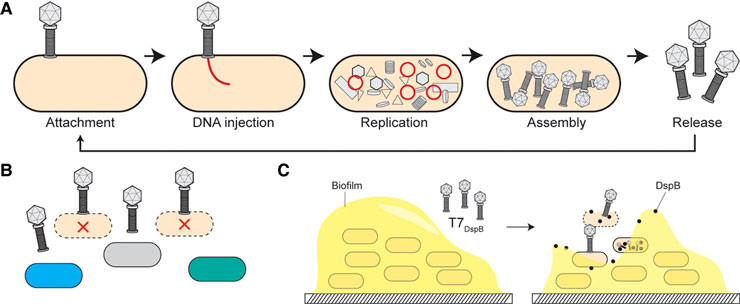
A) Phages, which are commonly present in the natural environment, infect bacteria by injecting their DNA into the cells, replicating inside them, and then exiting by killing their host.
B) For phage therapy, bacteriophages are isolated from the natural environment — for example, many of the phages used in aquaculture are obtained from the same ecosystems where the farmed species under study originate. The phages are administered to the infected animal, where the virus targets and destroys the bacteria responsible for the infection.
C) Phages can be genetically modified in the laboratory and used as vehicles to deliver lethal payloads to bacteria.
Proven applications of phages in aquaculture
In recent years, the use of phages in aquaculture has taken multiple forms – from direct injection to incorporating into feed. These therapies have been tested in both fish and invertebrates. Phages can be applied individually or as cocktails, and they serve both to improve animal health and to enhance food safety for consumers.
Most aquaculture-specific studies so far have focused on a limited number of species and on defining useful protocols for the prevention and treatment of bacterial diseases in fish farms. The best-studied pathogens to date include Streptococcus agalactiae, Aeromonas hydrophila, and Pseudomonas fluorescens – all of which can cause severe infections and high mortality in farmed tilapia (Oreochromis niloticus) and Euroean eel (Anguilla anguilla).
In tilapia, phages HN48 (Luo et al., 2018) and AP2 (Hassan et al., 2018) have been isolated and studied to combat S. agalactiae and A. hydrophila, respectively. According to these studies, tilapia infections could be resolved with a single injection of HN48, which might also serve as a prophylactic measure — offering fish farmers a valuable tool for disease control.
Another interesting example comes from Schulz et al. (2019), who tested a phage cocktail known as BAFADOR® against A. hydrophila and P. fluorescens infections in European eel (Anguilla anguilla).
This cocktail, administered via feed, contained three phages targeting A. hydrophila (50AhydR13PP, 60AhydR15PP, 25AhydR2PP) and four targeting P. fluorescens (22PfluR64PP, 67PfluR64PP, 71PfluR64PP, 98PfluR60PP). The treated eels showed improved health and significantly reduced mortality rates.
Phage therapy in fish
The effectiveness of phage therapy in fish had already been demonstrated in earlier studies on salmonids (trout and salmon), eel, catfish and olive flounder, against pathogens such as Aeromonas salmonicida, Edwardsiella tarda, Flavobacterium columnae, and Streptococcus iniae.
Similarly, in Senegalese sole (Solea senegalensis), the phage AS-A has been shown to be effective – and free from side effects – against A. salmonicida infections (Silva et al., 2016).
The main challenge now lies in moving from laboratory trials to farm-scale implementation. The first step involves developing effective protocols for incorporating bacteriophages into aquafeed.
One notable example is recently study published in Aquaculture by U.S. researchers, assessing the feasibility of including phages in feed formulations. The technique involves coating pellets with an edible, bactericidal layer – either by immersion in a serum or by spray application. According to the authors, this method keeps phages active during feed storage (Huang & Nitin, 2019).
Nevertheless, several challenges remain unresolved at the industrial level, particularly regarding variations in salinity, pH, and temperatures, which can affect or inactivate phages under real farming conditions.
Phage therapy in invertebrates
Recent scientific literature also highlights the benefits of phage therapy in several invertebrate’s species of major interest to aquaculture.
In sea cucumber (Apostichopus japonicus), for example, Ren et al. (2019) demonstrated the potential of a phage cocktail to act as a therapeutic agent against Vibrio parahaemolyticus infections.
Similarly, in Artemia franciscana, the application of phage cocktails to cysts improved hatching rates, increased survival, and reduced susceptibility of nauplii to Vibrio infections (Quiroz-Guzmán et al., 2018).
Phages as purification agents
Another promising application of phage therapy is its potential use in eliminating bacteria that compromise the food safety of commercially farmed bivalve molluscs – a method that could eventually be integrated into industry practices.
A study led by Le et al. (2018) with the Pacific oyster (Crassostrea gigas) applied a treatment using five phages specific to Escherichia coli and one specific to Salmonella. According to the results, incorporating phages into the production process “ensures the safety of a product that is usually consumed fresh, thus protecting consumer health”.
Biotechnology applied to phages
The use of phages as therapy rises several questions that researchers expect to resolve over time – particularly regarding biosecurity, biotechnology, and consumer perception of these biological agents.
So far, as reviewed by Dy et al. (2018), most phage research has been carried out using viruses isolated directly from the natural environments of the aquaculture species under study.
In future, biotechnology could employ to enhance their potential in both medicine and agri-food production. However, genetically modified viral therapies still have a long way to go, especially in aquaculture, where public acceptance of foods produced using such treatments may pose a challenge.
Another interesting avenue of research involves the use of inactivated phages. In this case, enzymes such as lysins – bactericidal proteins extracted from phages – can be modified in the laboratory to broaden their action against Gram-negative bacteria (Kropinski, 2018).
Encouraging results have already been obtained from studies with modified lysins. Although most work has been carried out in vitro, findings are promising and open up new lines of research (Dy et al., 2018; Cervantes et al., 2018).
Final considerations
In the near future, phages could become complementary or alternative treatments to antibiotics, helping to reduce their use and slow the emergence of resistant bacterial strains.
However, regarding food safety, further progress is still needed to clarify key aspects such as optimal administration methods, phage stability in the environment, and the safety of their use for both ecosystems and consumers.
References
Dy y colaboradores en BIOCHEMICAL SOCIETY TRANSACTIONS. 2018;46(6):1605-13.
Hassan y colaboradores en Journal of Pure and Applied Microbiology. 2018;12(3):1175-85.
Huang y colaboradores in Aquaculture. 2019;502:18-25.
Kropinski y colaboradores in Research in Microbiology. 2018;169(9):481-7.
Le y colaboredores en Current Microbiology. 2018;75(5):611-9.
Luo y colaboradores en Journal of Fish Diseases. 2018;41(10):1477-84.
Mateus y colaboradores en Aquaculture 424–425, 167–173.
Quiroz-Guzmán y colaboradores en Aquaculture. 2018;492:273-9.
Ren y colaboradores en Aquaculture. 2019;503:322-9.
Schulz y colaboradores en Fish and Shellfish Immunology. 2019;84:28-37.
Silva y colaboradores en Microb. Biotechnol. 7, 401–413.




